Riding the wave of capnography: Understanding ETCO2
Jaime Maher, CVT, VTS (ECC, Anesthesia/Analgesia)
Massachusetts Veterinary Referral Hospital, Woburn, MA
Posted on 2017-07-18 in Anesthesia & Analgesia
End-tidal carbon dioxide monitoring (ETCO2) has clinical uses far beyond solely determining hypo- or hyperventilation. It is the measurement of CO2 at the completion of exhalation and roughly correlates to the CO2 present in arterial blood. This non-invasive monitor can give valuable information about cardiac output, perfusion, and ventilation.
Aerobic cellular metabolism produces CO2 as a by-product. CO2 will then diffuse into venous circulation, be transported to the lungs and eliminated via exhalation. Anything that causes changes in circulation, tissue perfusion, metabolism, or ventilation can cause changes in CO2 production and elimination.
We often talk about the relationship or ratio between ventilation (V) and perfusion (Q) in regards to anesthesia (V/Q). Alveoli must be adequately ventilated but also adequately perfused in order for effective gas exchange to take place. Significant hypotension, for example, will cause inadequate perfusion although alveoli are still being ventilated. Atelectasis, bronchial intubation or lung pathologies will result in lack of alveolar ventilation although alveoli are perfused. We can evaluate this relationship using the gradient between ETCO2 and arterial CO2 levels (PaCO2) measured via arterial blood gas. In a normal, healthy patient this gradient, or difference, is minimal and the two are closely matched. The gradient can be reflected as P(a-ET)CO2, and the normal range for this is 2-5mmHg. As the gradient increases (PaCO2 is increasing while ETCO2 is decreasing or staying the same), this indicates that CO2 is not effectively being eliminated and is increasing at tissue levels. Poor perfusion (or increased alveolar dead space ventilation) is one common reason for this. If serial measurements are taken, a decrease in this gradient indicates that the patient’s status is improving. We also need to be cognizant of mechanical dead space (ETT length, monitor or positional adaptors, and anywhere that bidirectional gas flow occurs). Mechanical dead space will result in discrepancies between ETCO2 and PaCO2 as well.
Capnometry
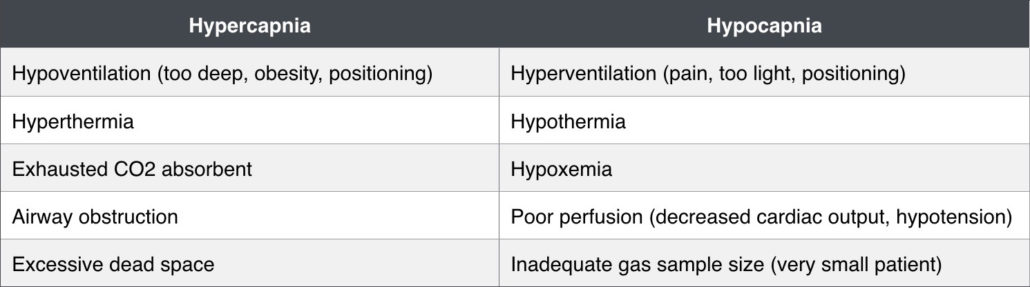
Capnometry is the measurement of carbon dioxide (CO2) in exhaled gas (ETCO2). This gives us a means of estimating ventilation and how well the lungs are removing CO2 from the body. Normal range is 35-45mmHg, and roughly correlates with the partial pressure of CO2 in arterial blood (remember that PaCO2 is usually slightly higher than ETCO2 by 2-5mmHg). We know that elevated ETCO2 (hypercapnia) occurs during hypoventilation, and a decrease in ETCO2 (hypocapnia) occurs with hyperventilation. Many capnographs also display a capnogram, or waveform which diagrams inspiration and exhalation over time. The capnogram can help to provide descriptive information about an abnormality.
There are two classifications of capnographs. Sidestream technology diverts sampled gases up a length of tubing to the monitor for infrared absorption analysis. Due to the diverting of gases, there is a slight delay in readings. Another drawback is that moisture can accumulate within the tubing causing an occlusion. In very small patients, some of the exhaled gas may become diluted with fresh gas resulting in a lower and inaccurate ETCO2 reading. In the presence of normal vitals and blood pressure, trends and capnogram waveforms are monitored more reliably than actual numbers in very small pets. Mainstream monitors have an infrared sensor that measures ETCO2 directly at the end of the endotracheal tube. These monitors provide quicker information as the gas is analyzed directly at end exhalation, and they do not require scavenging of the sampled gases. Mainstream monitors are larger and more cumbersome than sidestream and may not be appropriate for all patients.
Hypercapnia
Normal respiration is driven by CO2 levels in the blood stimulating respiratory centers in the brain. Many of the drugs that we use in anesthesia suppress this phenomenon. Patients are often unable to compensate for anesthetic related hypoventilation for example. As patients emerge from anesthesia, the respiratory drive becomes even more important because if it remains suppressed the elimination of inhalant is delayed. Some degree of hypercapnia during anesthesia may be tolerated in a spontaneously breathing patient without underlying disease (up to of 60mmHg). Manually ventilating too often in order to achieve a “normal” capnograph reading can cause the patient to rely on the anesthetist for ventilation. Assisting ventilation too aggressively will drive the PaCO2 down to a point where the patient will stop spontaneous ventilation. We do this intentionally sometimes in order to capture respirations when putting a patient on a mechanical ventilator or to stop the motion of breathing during certain imaging procedures.
Deep chested dogs, obese patients and patients positioned in dorsal recumbency are prone to hypoventilation and hypercapnia. Excessive dead space (endotracheal tube that is too long, adaptors between ETT and breathing circuit, sticking unidirectional valves) or exhausted sodalime can also contribute to hypercapnia. Mild to moderate hypercapnia can help stimulate the sympathetic nervous system and support heart rate (HR) and blood pressure (BP). If hypertension occurs during anesthesia, the patient is not hyperthermic and anesthetic depth seems adequate or increased, take note of the ETCO2. If this is elevated, decreasing the inhalant will often improve the patient’s ventilation and decrease both ETCO2 and BP.
Neurologic patients: An increase in PaCO2 will result in dilation of cerebral blood vessels allowing an increase in cerebral blood flow. This results in an increase in cerebral blood volume and intracranial pressure. Conversely, a decreased PaCO2 will decrease cerebral blood flow due to constriction of cerebral blood vessels. Ventilation should be controlled to maintain ETCO2 within low-normal limits in patients with potential neurologic disease (25-35mmHg).
Prolonged hypercapnia will lead to respiratory acidosis as there is a linear relationship between PaCO2 and pH. Generally speaking, a rise in PaCO2 of 10mmHg will result in a pH decrease of 0.1. As a patient experiences acidosis, we will also see an increase in K+ as there is an extracellular shift in potassium. This increase typically doesn’t result in clinically significant hyperkalemia but may be of importance in patients who are already hyperkalemic (i.e. cats with urethral obstructions). Mechanical ventilation may be warranted in these or otherwise acidotic patients.
Hypocapnia
Hypocapnia, or a decrease in ETCO2, can certainly indicate that the patient is hyperventilating (what is the patient’s respiratory rate?). Hyperventilation can occur when a patient’s anesthetic depth is not adequate, pain medication is needed, or the patient is being mechanically ventilated with an excessively high tidal volume or respiratory rate. However, since gas exchange is reliant on cardiac output and appropriate perfusion, hypocapnia can occur when blood flow is not adequate to perfuse alveoli. A sudden decrease or downward trend in ETCO2 can be an indicator of patient decline and imminent cardiac arrest. If the ETCO2 reading is suddenly zero, check the patient and anesthetic machine for a possible disconnect. We can also see artificially low ETCO2 readings in very small patients. The gas exhaled in these tiny pets may not meet the sample requirements for the monitor and/or the small sample may be diluted with fresh gas.
It is important to note that if a patient is experiencing apnea or the ETT is not properly placed (esophageal intubation) the ETCO2 will read zero as well. Capnography is therefore an excellent tool in verifying proper intubation and can, in fact, aid in guiding difficult intubations.
CPR
Because perfusion is necessary in addition to ventilation to remove CO2 from the body, capnography is very useful in determining the effectiveness of cardiopulmonary resuscitation. ETCO2 can be used to optimize chest compressions and detect return of spontaneous circulation (ROSC). A capnography monitor should be connected and ETCO2 should reach 10-20mmHg when performing chest compressions. If it is not, the manner in which chest compressions are being performed should be altered. A sudden increase in ETCO2 indicates ROSC.
Capnogram
The capnogram is a waveform displaying inspiration and exhalation over time. Crudely speaking, the upwards slope and top of the waveform is the exhalation phase, and the downwards slope and bottom of the waveform is the inhalation phase. The bottom of the waveform should reach zero, as no CO2 should be inhaled. The capnogram can provide a lot of information about equipment function as well as aid in explaining possible abnormalities. A few common abnormal capnograms include:
- Baseline does not return to zero during inspiratory phase: This indicates rebreathing of CO2 due to possibly exhausted soda lime, inadequate fresh gas flow, or a very small patient with a small exhaled gas sample being diluted in the sampling line (mixing of inhaled/exhaled gases).
- Elevation, or peak, at the end of exhalation just before inhalation (“shark fin”): This indicates an airway obstruction – check ETT length, check for kinks, check for mucus plug.
- Weak or “lazy” inhalation phase: Leak in the circuit – check ETT cuff and all connections.
- Regular “dips” in waveform during inhalation phase: Cardiogenic oscillations – can occur when respiratory rate is very slow and is the movement of gases at the ETCO2 adaptor consistent with the HR. This phenomenon is generally not of clinical significance.
ETCO2 monitoring can be challenging in very small patients, particularly those maintained on a non-rebreather. Even the smallest sidestream adaptor can add excessive dead space to the circuit, and mainstream monitoring is far too large and heavy for tiny ETTs. One trick that is useful, but only can be done with a sidestream monitor, is to insert a 22g needle into the ETT just after the circuit connector. The tubing from your sidestream monitor may then be attached directly to this needle, rather than adding an adaptor. Care must be taken when moving or repositioning the patient so as to not lose track of the needle. Once the patient is extubated, the ETT should be discarded unless it can be cut distally to the needle hole.
Dead Space
Dead space takes away from alveolar ventilation. Physiologic dead space is made up of our nasal passages, oropharynx, trachea, and some parts of the alveoli not being perfused. There is no gas exchange taking place in these areas. We estimate that physiologic dead space is 30-40% of tidal volume (approximately 3-5ml/kg). Normal tidal volume (Vt: the volume of gas that a patient inhales or exhales in one breath) is 10-20ml/kg.
Mechanical dead space is made up of any area in the anesthesia circuit that allows bidirectional gas flow. The patient end of the breathing circuit, positional adaptors, capnography adaptors, heat and moisture exchange devices, apnea monitors, and endotracheal tubes that extend past a patient’s incisors all contribute to mechanical dead space.

If we take an example of a 15ml/kg Vt on a 10kg patient, the Vt = 150ml. We then estimate that physiologic dead space is 4ml/kg (40ml); that will decrease the volume available for alveolar ventilation to 110ml. The patient is attached to an adult F-circuit (dead space = 8ml), ETCO2 adaptor (say, dead space = 5ml) and positional adaptor (dead space = 8ml). This leaves 89ml of Vt available for alveolar ventilation. If dead space is such that it equals Vt, then no CO2 will be able to be removed from the lungs.
Appendix
Further reading
- Bryant, Susan. 2010. Anesthesia for Veterinary Technicians. Wiley-Blackwell. pp 113-121
- Kodali, B.S., “Capnography in CPR”. www.capnography.com. 2012. Web. 22 Jan 2016.
- Kodali, B.S., “Oxygen and carbon dioxide pathways during anesthesia”. www.capnography.com. 2013. Web. 22 Jan 2016.
- Robertson, S. (2002) Oxygenation and Ventilation. In: Veterinary Anesthesia and Pain Management Secrets. (ed. S. Greene). pp 15-19. Hanley & Belfus, PA.
- Thurmon, Tranquilli, and Kurt Grimm. 2007. Lumb and Jones’ Veterinary Anesthesia 4th Edition. Williams, Wilkins, and Lippincott, Baltimore. pp 117-147, 547-549.
- Wilson, D. (2002) Respiratory Monitoring. In: Veterinary Anesthesia and Pain Management Secrets. (ed. S. Greene). pp. 113-119. Hanley & Belfus, PA.
About the author
|

 Jaime has been working as a certified veterinary technician for over 15 years. She is an active member of the Academy of Veterinary Technician Anesthetists and the Academy of Veterinary Emergency and Critical Care Technicians, through which she is recognized globally as a veterinary technician specialist in both anesthesia and emergency/critical care. Training and education are near and dear to Jaime’s heart, providing skilled and practical guidance as an anesthesia and ECC trainer, consultant and speaker. She is also a published author in ECC and anesthesia-related topics. In addition to working as a surgery and anesthesia technician at the Massachusetts Veterinary Referral Hospital, Jaime is also an instructor for Manchester Community College’s veterinary assistant course, getting back to basics for those interested in pursuing a career in veterinary nursing.
Jaime has been working as a certified veterinary technician for over 15 years. She is an active member of the Academy of Veterinary Technician Anesthetists and the Academy of Veterinary Emergency and Critical Care Technicians, through which she is recognized globally as a veterinary technician specialist in both anesthesia and emergency/critical care. Training and education are near and dear to Jaime’s heart, providing skilled and practical guidance as an anesthesia and ECC trainer, consultant and speaker. She is also a published author in ECC and anesthesia-related topics. In addition to working as a surgery and anesthesia technician at the Massachusetts Veterinary Referral Hospital, Jaime is also an instructor for Manchester Community College’s veterinary assistant course, getting back to basics for those interested in pursuing a career in veterinary nursing.