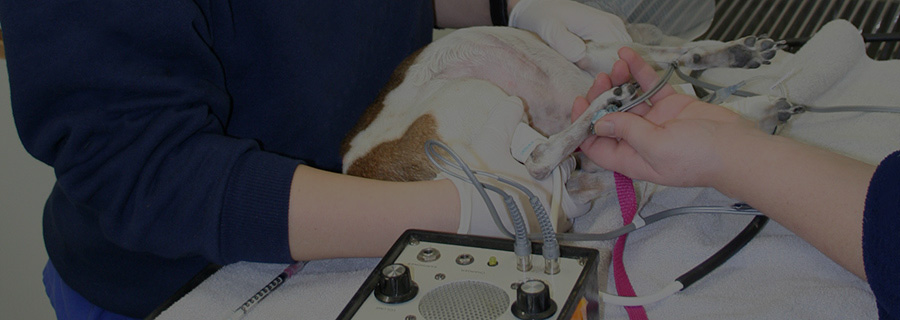
Taking indirect blood pressures
Stephen Cital RVT, SRA, RLAT
United Veterinary Specialty and Emergency, Oakland Zoo, San Francisco Zoo
Appropriate systemic arterial blood pressure is vital for survival in any species. In practice, we are faced with many reasons and conditions to obtain and interpret a patient’s blood pressure, such as anesthesia, cardiovascular disease and kidney disease. Both high and low blood pressure can be detrimental to our patients, so careful and accurate monitoring techniques are necessary. The two most common methods of non-invasive blood pressure (NIBP) measurement are Doppler ultrasound with a sphygmomanometer and oscillometry (Cardell or other machine). High definition (HD) oscillometry is a newer indirect technique and is becoming more common. Whenever taking a NIBP, it is important to note the trends of the data collected. NIBP is not an exact reflection of the patient’s true blood pressure, as there are many variables that can affect the results.
Doppler ultrasound measurement
Doppler ultrasound measurement is the most common blood pressure measurement technique used in small animal practice. In dogs, the reading attained most closely correlates to the systolic blood pressure, whereas in felines recent research is showing that it is more closely correlated to the mean arterial pressure (MAP). Some specialists will add 15 points to a Doppler derived pressure to better estimate the systolic pressure. Limitations when using the Doppler method include the user’s acute hearing, patient movement and cuff placement. A minimum of four readings should be taken to show a trend with the readings, discarding the first reading.
Supplies
Sphygmomanometer, blood pressure cuffs, ultrasound transmission gel, tape, clippers OR a NIBP machine
General tips
Blood pressure readings should be measured in a quiet, comfortable environment after the patient has become acclimated (i.e., without disturbance) for at least a few minutes, but acclimation is rarely possible for acutely or critically ill patients. Cats have good control of peripheral vasoconstriction and can become peripherally constricted under stress.
The patient should remain still in lateral recumbency during measurements to optimize accuracy. If necessary the patient can be restrained gently or have a towel placed over the head as a relaxation method. If the patient cannot be restrained in lateral recumbency because of agitation, respiratory distress, or other reasons, a standing BP can be obtained and a corrective equation used. Chemical restraint should be avoided.
The size of the cuff is important and should measure 40% of the appendage circumference in dogs and 30% in cats. Using a cuff that is too large will typically give falsely low blood pressure readings, while the opposite is true for a cuff that is too small.
Placement of the cuff is important. The most important thing is that the limb you use is at heart level whenever possible. Options for cuff placement when using the Doppler technique include mid-radius on the forelimb and proximal to the hock on the hindlimb. The base of the tail is also an effective site in small dogs and cats.
For oscillometric techniques, place the cuff mid-radius on the forelimb, the mid-tarsus on the hindlimb, or the base of the tail. The cuff tubing is placed over the artery on all locations and methods. The transducer tubing will ideally be directed away from the patient and towards the monitoring device. The cuff should be placed at the most even and cylindrical portion of the appendages.
To acquire the most accurate blood pressure values, cuff height off the floor or table should be as close to the level of the right atrium as possible.
Do not secure the cuff with tape tightly, as this will restrict airflow to the cuff’s bladder and cause inaccurate readings. A very loose piece can be used if absolutely necessary.
Do not place the cuff on a compromised limb or a limb with an arterial catheter, an IV catheter or a pulse Ox probe.
Technique
1. When using the Doppler unit, shave a small area on the posterior aspect of the paw, just above the largest pad on the front or hindlimb. If using the tail, shave a small area on the ventral aspect of the tail below where the cuff will be. When using the hindlimb, you can also shave and place the crystal over the more medial dorsal pedal artery. Apply a small amount of ultrasound conduction gel and use the Doppler crystal to find the clearest audible pulse. Secure the crystal head lightly with tape or hold still in place.
2. Using the sphygmomanometer, inflate the cuff until the pulse is no longer audible. Slowly release the pressure while listening for the first audible trace of the pulse to return and record this number. The first blood pressure measurement should be discarded.
3. Additional blood pressure measurements should be obtained for a minimum of 3 to 7 consecutive measurements. Consistent recordings with less than 20% variability are acceptable. Once these values have been obtained, they should be averaged to yield the blood pressure measurement.
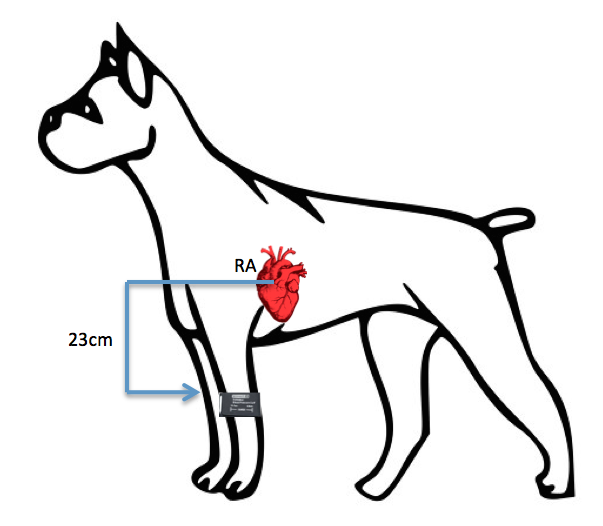 If the patient is sitting or standing and the height difference between the cuff and the right atrium exceeds 10cm, subtract 0.8mmHg for every 1cm the cuff sits below the right atrium.
If the patient is sitting or standing and the height difference between the cuff and the right atrium exceeds 10cm, subtract 0.8mmHg for every 1cm the cuff sits below the right atrium.
Example:
Initial systolic reading via Doppler or oscillometric reading was 160mmHg.
The top of the cuff is 23cm below the right atrium.
23cm X 0.8mmHg = 18.4mmHg
Corrected value:
160mmHg- 18.4mmHg = 141.6mmHg
If oscillometric machines are used repeat steps 1-3 and record readings. Use corrected value equation if patient is standing or sitting.
4. Once a pressure is taken, record the cuff size, patient positioning, appendage used and placement of crystal in the medical record for future readings. Keeping the method consistent is key.
Further reading
- Haberman CE, Kang CW, Morgan JD, et al. Evaluation of oscillometric and Doppler ultrasonic methods of indirect blood pressure estimation in conscious dogs. Can J Vet Res 2006;70:211-7.
- Jepson RE, Hartley V, Mendl M, et al. A comparison of CAT doppler and oscillometric Memoprint machines for non-invasive blood pressure measurement in conscious cats. J Feline Med Surg 2005;7:147-52.
- Meyer O, Jenni R, Greiter-Wilke A, et al. Comparison of telemetry and high-definition oscillometry for blood pressure measurements in conscious dogs: Effects of Torcetrapib. J Am Assoc Lab Anim Sci 2010;49:464-71.
- Pedersen, KM, Butler MA, Ersbøll AK, et al. Evaluation of an oscillometric blood pressure monitor for use in anesthetized cats. J Am Vet Med Assoc 2002;221:646-50.
- Sander C, Horauf A, Reusch C. Indirect blood pressure measurement in cats with diabetes mellitus, chronic nephropathy and hypertrophic cardiomyopathy. Tierarztl Prax Ausg K Kleintiere Heimtiere 1998;26:110-8.
- Stepien RL, Rapoport GS, Henik RA, et al. Comparative diagnostic test characteristics of oscillometric and doppler ultrasonographic methods in the detection of systolic hypertension in dogs. J Vet Intern Med 2003;17:65-72.
See also: Indirect blood pressure measurement