Stroke / infarct
Gena M. Silver, DVM, MS, DACVIM (Neurology)
Massachusetts Veterinary Referral Hospital, Woburn, MA
Posted on 2017-01-19 in Neurology
Cerebrovascular accidents (CVA), commonly termed “stroke,” are one of the major causes of disability and death in adult humans. Not so long ago considered uncommon in dogs and cats, with the use of MRI as the current standard of care for evaluation of veterinary neurological patients, we now realize that “strokes” do occur and actually are fairly common. The next 10 years will be gathering and assimilating information on causes, clinical presentation, breed predispositions, treatments, outcomes, and advances in imaging techniques to aid in accurate identification. The literature to date is evolving as this disease process becomes more routinely recognized.
The central nervous system requires a constant supply of glucose and oxygen to sustain its high expenditure of energy. There are five pairs of arteries that supply the brain. The first three supply the cerebral hemispheres (rostral, middle and caudal cerebral arteries), while the cerebellum is supplied by the rostral and caudal cerebellar arteries. These arteries arise from the circle of Willis with species variation determining the source of blood supplying this arterial circle.
A stroke occurs when there is a loss of brain function due to a disturbance in the blood supply to the brain. This disturbance can occur either due to ischemia (reduced or lack of blood flow) or hemorrhage. Therefore, stroke is broken down into two categories:
- Ischemic stroke
- Hemorrhagic stroke
Ischemic stroke
 The majority of strokes recognized in human and veterinary medicine are ischemic. They can be caused by either a local blockage of a blood vessel (thrombus) or a vascular obstruction transported to the brain from a distant site (thromboembolus). In humans, there are several classification systems depending on the vascular territory, the number of red blood cells that are found in the necrotic tissues dividing infarcts into pallid (pale) or hemorrhagic (red), the suspected underlying cause, and the size of the vessels involved. Disease processes involving large diameter blood vessels result in territorial infarcts, while diseases affecting smaller intraparenchymal penetrating arteries are responsible for lacunar infarcts. In humans, different disease processes are linked with different size vessel disease.
The majority of strokes recognized in human and veterinary medicine are ischemic. They can be caused by either a local blockage of a blood vessel (thrombus) or a vascular obstruction transported to the brain from a distant site (thromboembolus). In humans, there are several classification systems depending on the vascular territory, the number of red blood cells that are found in the necrotic tissues dividing infarcts into pallid (pale) or hemorrhagic (red), the suspected underlying cause, and the size of the vessels involved. Disease processes involving large diameter blood vessels result in territorial infarcts, while diseases affecting smaller intraparenchymal penetrating arteries are responsible for lacunar infarcts. In humans, different disease processes are linked with different size vessel disease.
Some reported causes of ischemic stroke in veterinary medicine are shown in the table at right. In approximately 50% of animals with ischemic stroke, the cause is undetermined. This is referred to a cryptogenic ischemic stroke.
Infarcts consist of a central core of severely hypoperfused tissue that dies rapidly and a surrounding region of tissue that has a potential to recover. This region is known as the penumbra and is the target for therapeutic interventions. The penumbra can also evolve into a larger area of infarction.
Hemorrhagic stroke
Hemorrhagic stroke is caused by bleeding from blood vessels of the brain either directly into the brain parenchyma or into the subarachnoid space. When the bleeding is substantial enough, the results can be fatal. In humans, the source of primary intraparenchymal hemorrhage is not completely understood, but patients often have systemic hypertension with concurrent fibrinoid degeneration of the arteries in the brain. Hypertension in dogs can be primary or secondary to systemic disorders, such as renal disease and hyperadrenocorticism. Secondary causes of hemorrhagic stroke described in dogs include hemorrhagic infarction, vascular malformation, neoplasia, vasculitis, and coagulopathies.
Diagnosis/Imaging
At one point, CT scan was the imaging modality of choice to evaluate for acute hemorrhagic infarcts in humans. With advances in technology, MRI is now the gold standard in evaluating for ischemic or hemorrhagic stroke, as well as ruling out other possible causes of brain dysfunction, including neoplasia, trauma or inflammation.
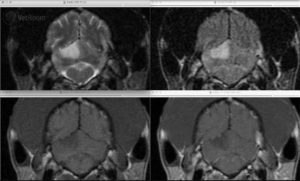
Fig. 1: Transverse MRI images of a cerebellar infarct in a Greyhound. There is a wedge-shaped lesion in the dorsal cerebellum that is hyperintense on T2-weighted (upper left) and FLAIR images (upper right), hypointense on pre-contrast T1-weighted images (lower left), and does not contrast enhance (lower right). Image courtesy of Dr. Mark Troxel, Massachusetts Veterinary Referral Hospital.
Ischemic strokes appear iso- to hypointense on T1-weighted images and hyperintense on T2-weighted and FLAIR sequences (fig. 1). Enhancement is not seen until reperfusion is noted 7-14 days following the stroke and at this time is faint and on the periphery of the lesion. Enhancement along with the lesion tends to resolve by 6-8 weeks post-injury.
More recent advances have included the use of diffusion-weighted imaging (DWI; fig. 2) and ADC mapping. Acute infarction results in water trapping within cells and causes reduced diffusion, which is seen as increased signal on the DWI. Some other lesions that are hyperintense on T2 will also be hyperintense on DWI, which is called “T2 shine through.” To differentiate this from true restricted diffusion from ischemic stroke, an ADC can be calculated. Areas with restricted diffusion will have low ADC values and will appear as a hypointensity on most ADC maps. Abnormalities on DWI can be seen within one hour of onset of ischemia with a reduction in ADC seen within 2.5 minutes. The classic appearance of acute infarction is hyperintensity of the DWI and hypointensity on the ADC. This pattern persists for the first 4 days and then the ADC will start to normalize from 4-10 days. The DWI can stay hyperintense for 72 days post-infarction.
In human MRI, perfusion weighted imaging (PWI) is used to identify regions of hypoperfusion (penumbra). In theory, this alerts the physician to cases that will benefit from thrombolytic treatment even beyond the typical therapeutic window.
Magnetic resonance angiography (MRA), commonly used in humans, is in the infancy stage in veterinary medicine. Understanding what we can achieve and see in the normal dog and cat will be required before we can accurately interpret abnormal scans.
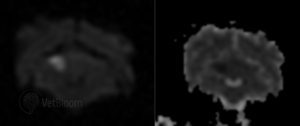
Fig. 2: Transverse DWI and ADC map from a dog with a right cerebellar infarct. Note the hyperintense wedge-shaped lesion on the DWI (left) that is hypointense on the ADC map (right). Image courtesy of Dr. Mark Troxel, Massachusetts Veterinary Referral Hospital.
Although DWI and ACD mapping are now commonly used in veterinary medicine and have greatly improved the ability to identify acute ischemic infarcts, the size of our patients and limited experience makes PWI and MRA still an area of “maybe in the future.”
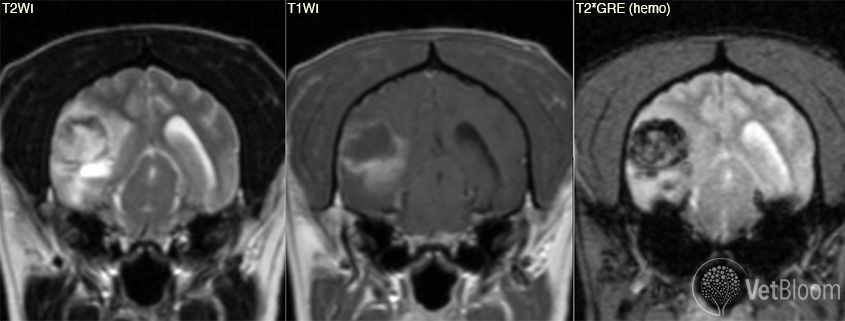
The MR image appearance of hemorrhage is dependent on the age of the bleed. As blood is broken down from oxyhemoglobin to deoxyhemoglobin, methemoglobin and then hemosiderin, each phase has different paramagnetic properties. The gradient-echo sequence is the most accurate of all of the MRI sequences and is more accurate than CT in predicting the presence and extent of hemorrhage. Hemorrhage, regardless of its age, will appear as a hypointensity on the GRE. Hypointensity on gradient echo is not specific for hemorrhage and can be seen with air, calcification, iron, melanin and foreign bodies. History and appearance of the lesion on other sequences will enable the reviewer to determine if the lesion is due to hemorrhage.
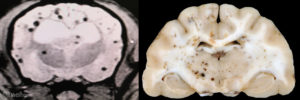
Fig. 3: Transverse MRI and gross pathology images from two dogs with cerebral microbleeds. These are frequently incidental findings when the patient is imaged for other reasons. MRI courtesy of Dr. Eric Glass, Red Bank Veterinary Hospital. Gross pathology courtesy of Dr. Andrew Miller, Cornell University.
Treatment
Once the diagnosis of stroke is made, an attempt to identify the underlying cause is initiated. Evaluation for hypertension (and its causes), endocrine disease (hyperadrenocorticism, hypothyroidism, hyperthyroidism, diabetes mellitus), kidney disease (protein-losing nephropathy), heart disease and metastatic disease is performed. If a hemorrhagic stroke was identified, an evaluation for hypertension (and its causes), a coagulopathy and metastatic disease should be performed. If a cause of stroke is identified, treatment is initiated.
Ischemic stroke
Treatment for stroke in people involves three steps.
Restoration and monitoring of vital homeostatic function
In animals, the current recommendation is primarily around restoration and monitoring vital homeostatic functions and maintaining systemic blood pressure and normal ventilation to preserve the tissue in the ischemic penumbra. If blood pressure is consistently above 180 mmHg, antihypertensives are recommended; however, aggressive lowering should be avoided as hypertension can occur as a physiological response to a stroke to ensure adequate CPP in the penumbra of the infarct for up to 72 hours.
Initiation of neuroprotection
There is no evidence that glucocorticoid treatment provides neuroprotection in cases of stroke. Other neuroprotective agents such as NMDA antagonists, calcium channel blockers, sodium channel modulators, and hyperbaric oxygen therapy have not proven to be clinically helpful in human or animal stroke patients.
Thrombolysis
- Unfractionated heparin: No data in humans or animal to confirm a significant improvement in clinical outcome in patients with acute ischemic stroke.
- Recombinant Tissue Plasminogen Activator (rTPA): Used in human stroke patients if it can be administered within the first three hours. It will result in an overall benefit of 10% with respect to living without disability. It does not improve chances of survival. A study in 2014 noted a 5% increase in the number of people living without disability at 3-6 months; however, there was a 2% increased risk of death in the short term. Benefits are greater when given earlier, especially within 3 hours. The benefit is unknown if given 3.0-4.5 hours later, and outcomes worsen if given after 4.5 hours.
- Anti-platelet therapy with low-dose aspirin (0.5mg/kg q 24 hrs) or clopidogrel (2-4 mg/kg q24h) can be used prophylactically to prevent clot formation in proven cardiac sources of emboli.
Hemorrhagic Stroke
The most important aspect of treatment is to identify and treat the underlying cause if one can be determined. Serial neurologic examinations to identify signs of increased intracranial pressure. Supportive care should include maintenance of PaCO2 within normal range, not to exceed 40 mmHg, with intubation if needed.
Systemic blood pressure should be corrected and monitored to ensure cerebral perfusion pressure is maintained. Hypertonic saline (dog: 4-6 mL/kg; cat: 2-4 mL/kg; over 5-10 min) is preferred to achieve rapid restoration of blood volume and pressure while limiting the volume of fluid administered. As with ischemic stroke, hypertension should be treated if consistently > 180 mmHg.
Mannitol (0.25-2 g/kg IV over 10-20 min) is used to treat increased intracranial pressure secondary to hemorrhagic stroke by improving cerebral blood flow by reducing blood viscosity.
Surgery to eliminate the space occupying mass if the hematoma is subarachnoid and the patient is deteriorating neurologically.
Prognosis
The prognosis for ischemic or hemorrhagic strokes depends on the initial severity of the neurological signs. Most cases of ischemic stroke will recover within weeks with supportive care. One study of a small number of dogs (33) report that dogs with concurrent medical conditions had a shorter survival time and were likely to suffer from subsequent infarcts than those dogs with no identifiable medical condition.
Further reading
- Altay UM, Skerritt GC, Hilbe M, et al. Feline cerebrovascular disease: Clinical and histopathologic findings in 16 cats. J Am Anim Hosp Assoc 2011;47:89-97.
- Axlund TW, Isaacs AM, Holland M, et al. Fibrocartilaginous embolic encephalomyelopathy of the brainstem and midcervical spinal cord in a dog. J Vet Intern Med 2004;18:765-7.
- Barton-Lamb AL, Martin-Flores M, Scrivani PV, et al. Evaluation of maxillary arterial blood flow in anesthetized cats with the mouth closed and open. Vet J 196(2013)325-33.
- Cervera V, Man W, Vite CH, et al. Comparative magnetic resonance imaging findings between gliomas and presumed cerebrovascular accidents in dogs. Vet Rad Ultrasound, 2011;52:33-40.
- de Lahunta AD, Glass EN, Kent M. Veterinary Neuroanatomy and Clinical Neurology 4th ed. Elsevier 2015
- Evans HE, de Lahunta AD. Miller’s Anatomy of the Dog 4th ed. Saunders 2012.
- Fulkerson CV, Young BD, Jackson ND, et al. MRI characteristics of cerebral microbleeds in four dogs. Vet Radiol Ultrasound 2012;53:389-93.
- Garosi LS, McConnell JF. Brain infarct in dog and humans: a comparative review. J Small Anim Pract 2005;46:521-9.
- Garosi LS, McConnell JE, Platt SR, et al. Results of diagnostic investigations and long-term outcome of 33 dogs with brain infarction (2000-2004). J Vet Intern Med 2005;19:729-31.
- Garosi LS, McConnell JF, Platt SR, et al. Clinical and topographical magnetic resonance characteristics of suspected brain infractions in 40 dogs. J Vet Intern Med 2006;20:311-21.
- Garosi LS. Cerebrovascular disease in dogs and cats. Vet Clinic North Am Small Animal Pract 2010:40:65-79.
- Glass EN, Cornetta AM, deLahunta A, et al. Clinical and clinicopathological features in 11 cats with Cuterebra larvae myiasis of the central nervous system. J Vet Intern Med 2008;12:365-8.
- Gredal H, Toft N, Westrup U, et al. Survival and clinical outcome of dogs with ischaemic stroke. Vet J 2013;196:408-13.
- Irwin JC, Dewey CW, Stefanacci JD. Suspected cerebellar infarcts in 4 dogs. J Vet Emerg Critic Care 2007;17:268-74.
- Kent M, Glass EN, Haley AC, et al. Ischemic stroke in Greyhounds: 21 cases (2007-2013). J Am Vet Med Assoc 2014;245:113-17.
- King AS. Arterial supply to the central nervous system. In: King AS, ed. Physiological and clinical anatomy of the domestic mammals: central nervous system, volume 1 Oxford: Oxford University Press, 1987;1-12
- Lowrie M, De Risio L, Dennis R, et al. Concurrent medical conditions and long-term outcome in dogs with non-traumatic intracranial hemorrhage. Vet Radiol Ultrasound 2012;53:381-8.
- McConnell JF, Garosi L, Platt SR. MRI findings of presumed cerebellar cerebrovascular accident in twelve dogs. Vet Radiol Ultrasound 2005;46:1-10.
- Platt SR, Garosi L. Cerebrovascular Accidents, In: Platt SR, Garosi L. Small Animal Neurological Emergencies. Manson Publishing, 2012;319-339.
- Platt SR, Garosi L. Canine cerebrovascular disease: Do Dogs have strokes? J Am Anim Hosp Assoc 2003;39:337-42.
- Rylander H, Eminaga S, Palus V, et al. Feline ischemic myelopathy and encephalopathy secondary to hyaline arteriopathy in five cats. J Feline Med Surg 2014;16:1-8.
- Gredal H, Skerritt GC, Gideon P, et al. Spontaneous ischaemic stroke in dogs: Clinical topographic similarities to humans. Acta Neurol Scand 2013;128:e11-6.
- Theobald A, Volk HA, Dennis R, et al. Clinical outcome in 19 cats with clinical and magnetic resonance imaging diagnosis of ischaemic myelopathy (2000-2011). J Feline Med Surg 2013;15:132-41.
- Vandevelde M, et al. Veterinary Neuropathology: Essentials of Theory and Practice. Wiley-Blackwell 2012.
- Wolff CA, Holmes SP, Young BD, et al. Magnetic resonance imaging for the differentiation of neoplastic, inflammatory, and cerebrovascular brain disease in dogs. J Vet Intern Med 2012;26:589-97.
- Yoshinarki K, Garcia JH. Carotid arterial supply of the feline brain. Applications to the study of regional cerebral ischemia. Stroke 1975;6:361-9.
About the author
Dr. Silver gained board-certification in neurology by the American College of Veterinary Internal Medicine in 2000. Dr. Silver is a member of the American College of Veterinary Internal Medicine, American Veterinary Medical Association, Massachusetts Veterinary Medical Association, and is an active board member on the Veterinary Association of the North Shore. Dr. Silver has taken the neurosurgical certification course offered by ACVIM in 2012 and a neurosurgical stabilization course offered by AO North America in 2013. Dr. Silver has lectured for MVMA, VANS, and many in house CE lectures for hospitals in the IVG network of hospitals. She has hosted veterinary students, interns and residents interested in additional experience neurology/neurosurgery. Enjoying all aspects of neurology, Dr. Silver has a special fondness for disk disease in all animals! |

 Dr. Silver has been at Massachusetts Veterinary Hospital since its opening in 2000, developing the neurology service from the ground up. She is from Nova Scotia, Canada and earned her veterinary degree from the Atlantic Veterinary College in Prince Edward Island, Canada in 1995. She then completed a small animal medicine and surgery internship at Washington State University where she remained for a three-year residency in neurology and neurosurgery. Following the residency, she stayed on faculty for a one-year clinical instructor position in neurology and neurosurgery. Washington State University was the first veterinary hospital in the world that had an in-house MRI unit, giving Dr. Silver more than 16 years of experience in using MRI as the primary imaging modality for neurologic patients.
Dr. Silver has been at Massachusetts Veterinary Hospital since its opening in 2000, developing the neurology service from the ground up. She is from Nova Scotia, Canada and earned her veterinary degree from the Atlantic Veterinary College in Prince Edward Island, Canada in 1995. She then completed a small animal medicine and surgery internship at Washington State University where she remained for a three-year residency in neurology and neurosurgery. Following the residency, she stayed on faculty for a one-year clinical instructor position in neurology and neurosurgery. Washington State University was the first veterinary hospital in the world that had an in-house MRI unit, giving Dr. Silver more than 16 years of experience in using MRI as the primary imaging modality for neurologic patients.