It’s getting harder and harder to breathe: How I treat pneumonia
Katie Sakakeeny, DVM, DACVECC
IVG Metrowest Veterinary Referral Hospital, Natick, MA
Posted on 2017-05-30 in Emergency & critical care, Internal medicine
Pneumonia is defined as an infection within the lungs. More specifically, pneumonia is an infection that develops at the level of the alveoli and may affect multiple lobes. Bacterial pneumonia is most commonly described, although viral, fungal, and even parasitic causes of pneumonia are possible. Bacterial pneumonia is most commonly encountered among our patients and is further classified into primary infectious bronchopneumonia or aspiration pneumonia. Depending on the underlying etiology, treatment can vary. Although reported, feline bacterial and viral pneumonia are considered rare so most of the information summarized will pertain to the canine population.
Clinical signs and physical examination findings
The most common complaints by owners include lethargy, anorexia, cough, nasal discharge, and dyspnea. Vomiting and/or regurgitation is not always described (or witnessed) in cases of aspiration pneumonia. In some cases, thorough questioning of an owner is crucial to discover whether “silent regurgitation” may be occurring in the absence of actual productive retching. This is especially important to ask in cases of brachycephalic pneumonia. In some cases, lethargy and/or anorexia alone may be the only clinical signs described. Pneumonia can also be an incidental finding when a patient is getting worked up for a different problem and has no clinical signs consistent with pneumonia. In cases of suspected infectious bronchopneumonia, a thorough history should be obtained about recent exposure to other dogs and travel history.
Several studies have been performed and identified risk factors for aspiration pneumonia. Among these include recent vomiting or regurgitation (most commonly reported), recent anesthesia, esophageal disease or dysfunction, laryngeal disease, and neurologic disease. Brachycephalic breeds are more prone to aspiration pneumonia due to their conformation and decreased ability to protect their airway. They also commonly have GI motility disorders that can lead to frequent regurgitation, which adds to this risk.
The most common physical exam findings include fever, harsh or increased bronchovesicular sounds, pulmonary crackles, dyspnea, tachypnea, cough, nasal and/or ocular discharge. These findings do not always have to be present, however, and often times one or two symptoms are described. In contrast to harsh bronchovesicular sounds or crackles, patients can alternatively have absent, dull, or normal lung sounds. In cases of complete alveolar consolidation, absent lung sounds are common.
Diagnostics
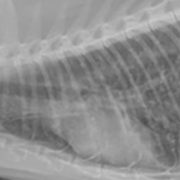
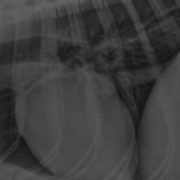
The most commonly used diagnostic tool for diagnosing pneumonia is the radiograph. For bronchopneumonia, there is no single lung pattern that is pathognomonic. Alveolar, interstitial, and mixed alveolar-interstitial patterns are the most common. They can be focal, patchy, or diffuse in distribution. The classic radiographic sign for aspiration pneumonia is an alveolar pattern or consolidation of the right middle lung lobe. However, ANY lung lobe(s) can be affected in cases of aspiration, and while an alveolar pattern tends to be more representative of aspiration, interstitial patterns can also be seen, especially with early stages of aspiration. Clinical signs do not necessarily correlate with severity of pulmonary infiltrates on radiographs, so radiographs alone should not be used to grade the severity of disease or to prognosticate. Radiographs should be withheld in cases where a patient is too unstable and at risk of cardiac arrest in radiology. Providing oxygen support +/- sedation and antibiotics is warranted to help stabilize the patient before obtaining imaging.
A minimum database (CBC, serum chemistry profile, blood gas, and urinalysis) is ideal in any situation where a patient is or could become critically ill as a baseline for comparison, although it is not entirely necessary for every patient with suspected pneumonia, and in cases of severe distress, this may not be practical until oxygen support and stabilization are employed. Typical CBC findings for pneumonia include a neutrophilia +/- a left shift, although a normal leukogram, as well as leukopenia, can also be seen. A chemistry profile is helpful if sepsis is suspected (which can reveal an elevated bilirubin, hypoglycemia, and/or hypoalbuminemia) or to determine if there are concerns with liver or kidney function, which can be important when choosing antibiotics. Hypoalbuminemia was a commonly reported finding in one study evaluating dogs with aspiration pneumonia. A baseline urinalysis is ideal in the event that aminoglycoside therapy is employed to ensure that there is no pre-existing renal dysfunction and to provide a comparison for future urinalyses to look for casts once therapy has been initiated. An arterial blood gas may be the most useful quantitative tool when there is concern that a patient may require mechanical ventilation. Arterial blood gas changes will typically show a degree of hypoxemia (defined as a PaO2 < 80 mm Hg on room air) +/- hypercapnea (PCO2 > 45 mm Hg), although actual reports of hypercapnea from pneumonia is rare. More commonly I’ve found hypoxemia with hyperventilation (PCO2 < 35 mm Hg) which indicates that a patient is working hard to get oxygen and may be expelling CO2 faster than they are absorbing oxygen. Arterial blood gases can be technically difficult and distressing to a dyspneic patient. Unfortunately, the most dyspneic patients are the ones in which an arterial blood gas provides the most useful information on whether MV is indicated. In cases where a patient becomes too stressed while trying to obtain a blood gas, pulse oximetry can be considered as an alternative but is not necessarily as accurate as falsely low readings are common. An SpO2 of < 93% is considered hypoxemic, although I tend not to get very concerned until a patient has dropped to 90% or lower as a risk for ventilation. Bottom line- if a patient is too distressed to obtain a blood gas or pulse oximetry reading safely, then mechanical ventilation is likely indicated and referral to a hospital where ventilation is offered would be recommended. In cases where a blood gas is successfully obtained, a PaO2 < 50-60 mm Hg while receiving oxygen supplementation (typically between 40-50% FiO2) is considered an indication for ventilation. A PCO2 >50 is also considered an indication for ventilation. Despite quantitative criteria, the decision to ventilate can be perceived as a tough decision to make as a clinician. The most important advice I was given in my training in regards to ventilation is “if you are debating about whether your patient may need mechanical ventilation, they probably need it.”
Airway sampling in the form of bronchoalveolar lavage (BAL) and endotracheal washing (ETW) are among the additional yet more invasive diagnostics available to obtain information at the cellular level and to obtain cultures. Their value is especially important in cases where bronchopneumonia vs. aspiration pneumonia cannot be determined. They are also strongly recommended in cases of hospital-acquired pneumonia, in patients with a previous history of pneumonia, and when a patient is not responding to antibiotic therapy as predicted.
BAL involves the use of a bronchoscope to guide a clinician directly into the lower airways to obtain samples. The benefit of this technique is actual visualization of the lower airways in real time so that a representative sample can be obtained. It also can provide a look at the upper airway as well (i.e., in case there are concerns about tracheobronchomalacia or rhinitis) and thus be used for multiple diagnostic purposes. In large breed dogs, this technique may be preferred over ETW (to be discussed below) given that BAL is more likely to achieve true lower airway samples and can verify this with a scope, while ETW is a fairly blind procedure where retrieval of lower airway samples vs distal tracheal samples is not always certain and is assumed. In smaller animals where a scope cannot fit inside an ET tube, BAL can be risky as the scope may have to be advanced without a protected airway. While oxygen can flow through the scope, many patients are already respiratory compromised at the time of this procedure and may have difficulty with oxygenating without the constant presence of an ET tube connected to 100% oxygen. Patient selection for BAL should be carefully considered as a result.
Endotracheal washing (ETW) is another valuable tool in pursuing a diagnostic work-up of pneumonia and is less time-consuming, slightly less invasive, does not require the same level of expertise, and is more cost effective than BAL. An ETW is performed by performing sterile endotracheal intubation and instilling a small amount (usually between 6-20 ml) of sterile NaCl through the ET tube into the lower airway. Someone other than the “intubator” then coupages the patient vigorously while the intubator suctions the instilled fluid using sterile technique. The suction catheter is removed, and the patient is then connected to 100% oxygen and monitored closely. The process requires heavy sedation, but when done correctly should take only about 5 minutes or less from start to finish. It requires a minimum of 3 people to perform (one performing sterile intubation, instillation, and suction; one performing coupage; one performing sedation monitoring and assisting with supplies). The risks involved with this procedure are obvious; heavily sedating an already dyspneic patient carries the risk for respiratory and cardiac arrest, and further risk is assumed when fluid is instilled into an already compromised airway. The total fluid instilled into the airway is not completely suctioned back, so a patient now has additional fluid in their airway that they did not have prior to the ETW. Careful monitoring of the patient should occur not only during the wash but on recovery and following extubation for several hours. These risks should be communicated with owners and documented prior to obtaining their consent for this procedure. Once a fluid sample is obtained, a sample should be submitted for aerobic culture and sensitivity and another sample should be submitted for fluid analysis and cytology. In-house cytology should also be performed as a preliminary screening to help in guiding antibiotic therapy while awaiting final culture results. In cases of suspected infectious bronchopneumonia, an additional canine respiratory disease panel is recommended. The panel we currently use through an outside lab tests for bordetella, mycoplasma, distemper, influenza, coronavirus, herpesvirus, adenovirus, among others.
Because ETW’s carry an inherent risk and can be costly to owners, the risks vs. benefits should always be weighed carefully before deciding to proceed. For example, a first-time, severely dyspneic, suspected aspiration pneumonia that occurred at home does not necessarily mandate an ETW for a bacterial culture as there should be a predicted response to the common broad spectrum antibiotics employed for a first-time, non-resistant aspiration pneumonia. Alternatively, a dog with his third episode of aspiration pneumonia following a laryngeal tieback procedure should absolutely be considered for an ETW as long as he is not too unstable for the procedure. Another scenario in which an ETW should be recommended is in cases of “shipping pneumonia”, where a puppy is shipped from a distant location and presents to you with presumed infectious bronchopneumonia, although aspiration pneumonia may not be completely ruled out. Due to different bacterial and viral strains that can vary across regions, and due to concerns for reportable diseases (such as canine influenza and distemper), the information gained from performing a wash in this scenario may be important from a public health perspective.
Treatment
 Oxygen supplementation is the most obvious and important initial form of treatment for patients who are dyspneic from pneumonia. However, not all patients who are diagnosed with pneumonia are truly oxygen-dependent, and supplementation can be a subjective decision based on the primary clinician’s judgment or based on documented hypoxemia in the form of an arterial blood gas or pulse oximetry. The most common forms of oxygen supplementation include flow-by, face mask with flow-by, an oxygen hood, nasal oxygen lines, and an oxygen chamber/cage. With flow-by, typical oxygen supplementation can be hard to quantify and can be as low as 25% (only 4% higher than room air) when set at a flow rate of 2-3 L/min. When flow-by is combined with a tight-fitting mask and the flow rate is increased to 8-10 L/min, the FiO2 can increase to 40-60%. An oxygen hood can be assembled using cling wrap and an E collar with oxygen tubing slid under the collar coming from a caudal to cranial direction. Flow rates of 1 L/min generally will achieve an FiO2 of 30-40%. This can serve as a temporary make-shift oxygen chamber, but not all patients will tolerate this, and if there is no room left for venting, carbon dioxide can accumulate within the hood if left in place for many hours. An oxygen chamber, depending on the type, can administer up to 60% FiO2. The added benefit of a chamber is that they are environment-controlled for humidity, temperature, and they provide ventilation to prevent CO2 accumulation. However, these units can be costly and require specific maintenance and may not be practical for all hospitals.
Oxygen supplementation is the most obvious and important initial form of treatment for patients who are dyspneic from pneumonia. However, not all patients who are diagnosed with pneumonia are truly oxygen-dependent, and supplementation can be a subjective decision based on the primary clinician’s judgment or based on documented hypoxemia in the form of an arterial blood gas or pulse oximetry. The most common forms of oxygen supplementation include flow-by, face mask with flow-by, an oxygen hood, nasal oxygen lines, and an oxygen chamber/cage. With flow-by, typical oxygen supplementation can be hard to quantify and can be as low as 25% (only 4% higher than room air) when set at a flow rate of 2-3 L/min. When flow-by is combined with a tight-fitting mask and the flow rate is increased to 8-10 L/min, the FiO2 can increase to 40-60%. An oxygen hood can be assembled using cling wrap and an E collar with oxygen tubing slid under the collar coming from a caudal to cranial direction. Flow rates of 1 L/min generally will achieve an FiO2 of 30-40%. This can serve as a temporary make-shift oxygen chamber, but not all patients will tolerate this, and if there is no room left for venting, carbon dioxide can accumulate within the hood if left in place for many hours. An oxygen chamber, depending on the type, can administer up to 60% FiO2. The added benefit of a chamber is that they are environment-controlled for humidity, temperature, and they provide ventilation to prevent CO2 accumulation. However, these units can be costly and require specific maintenance and may not be practical for all hospitals.
Early and appropriate antimicrobial therapy is also essential for successful treatment of pneumonia. This becomes challenging in cases where infectious bronchopneumonia vs. aspiration pneumonia cannot be determined as the antibiotic choices for each condition vary and can carry a variety of side effects depending on the patient. With aspiration pneumonia, the most common pathogen reported is E. Coli. With primary infectious pneumonia, Bordetella is most commonly isolated (with or without Mycoplasma. Mycoplasma is not routinely cultured in laboratories and its overall prevalence may not be known in all cases). (Note: there is minor variability among studies with additional organisms such as Staph, Strep, Klebsiella, Pasteurella among others reported in higher frequency for different types of pneumonia. I have oversimplified the interpretation of reported culture results here but provided a table summarizing the cultures from the literature below).
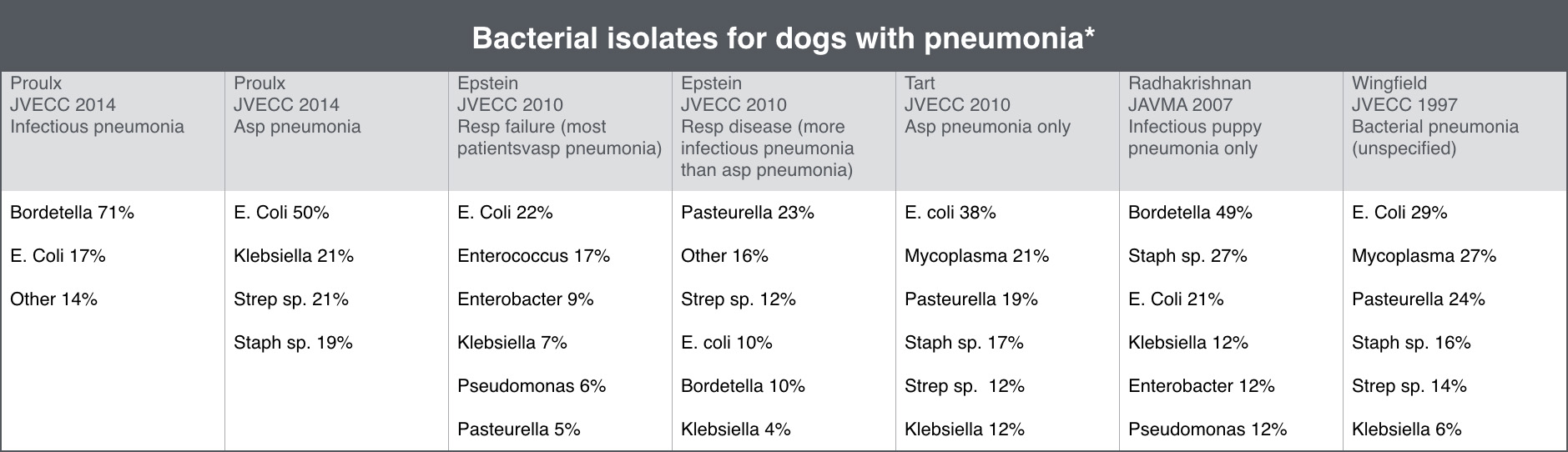
*Percentages may not equal 100% because some patients grew more than one bacterium per culture and some patients grew smaller numbers of other bacteria that were excluded from this table (only the top 3-6 types of isolates are included in this table).
Recently a consensus statement was published for antibiotic selection for aspiration vs. bronchopneumonia, with the rationale being that the antibiotic selection should be targeted toward the most likely pathogens infecting the patient. This may be a judgment call made by the attending clinician based on their suspicion for whether this is bronchopneumonia, aspiration pneumonia, or another cause of pulmonary disease. There are studies that have shown that inappropriate initial antibiotic selection for pneumonia in people carries a significantly higher mortality rate than those whose initial antibiotic regimen was appropriate. In cases where it truly is difficult to know whether aspiration or bronchopneumonia is more likely, airway sampling either in the form of ETW or BAL would be recommended. The most commonly prescribed antibiotics for pneumonia include amoxicillin/clavulanate, doxycycline, and fluoroquinolones. Based on culture results obtained from multiple studies, these choices appear to be reasonable for first-time or community-acquired forms of pneumonia. In cases of hospital-acquired pneumonia, or pneumonia that develops while currently being treated with antibiotics, resistance to these antibiotics is common, and airway sampling for cytology and culture is recommended to guide therapy. In cases of aspiration pneumonia, amoxicillin/clavulanate may be sufficient for many first-time infections, although many clinicians will combine this therapy with enrofloxacin for broader spectrum (i.e., against Pseudomonas and Enterobacter) and because enrofloxacin is more effective at penetrating respiratory tissue than penicillins. The use of amoxicillin with clavulanate (Clavamox/Augmentin) is quite common among clinicians, and many specialists believe it is an adequate first-line choice for most of the common pathogens that cause pneumonia. The efficacy of Clavamox is controversial in treating Bordetella, and given the multiple sources that document doxycycline and chloramphenicol as effective treatments for Bordetella, I will usually choose one of these over Clavamox when I am highly suspicious of Bordetella. In possible aspiration pneumonia, Clavamox may be the best available first-line choice (again +/- enrofloxacin added). Suggested doses for pneumonia tend to be higher than for other types of infections (20 mg/kg TID x 8 wks; Morgan, 2011 Small Animal Drug Handbook). This is a much higher and more frequent dosing interval than most clinicians typically prescribe. However, given the concern about effective respiratory penetration even in times of inflammation/disease, higher doses, and increased frequency may be warranted, although there are no studies to my knowledge that have actually proven this. Along these lines, I choose to use high doses of ampicillin-sulbactam (virtually the injectable equivalent of Clavamox) at 50 mg/kg IV q 8 hr for pneumonia rather than the standard 22-30 mg/kg q 8 hr dose. However, since the recent consensus statement was published from ACVIM, the experts on this panel have lower doses recommended for ampicillin-sulbactam (20 mg/kg IV, IM q 6-8 hr) and amoxicillin-clavulanate (dogs 11 mg/kg PO q 12 hr; cats 12.5 mg/kg PO q 12 hr).
For infectious pneumonia, doxycycline (5 mg/kg q 12 hr) and chloramphenicol (50 mg/kg q 8 hr) were previously my top 2 choices. The Working Group from the ACVIM Consensus Statement recommends using chloramphenicol as a drug reserved only for multidrug-resistant infections or when an owner cannot afford dual treatment for possible aerobic and anaerobic infections. I reserve enrofloxacin for adult dogs with suspected infectious pneumonia if they do not respond in 2-3 days as expected to alternative antibiotics or in patients where they may have significant hepatic disease as both doxycycline and chloramphenicol can cause liver enzyme elevation and hepatotoxicity (enrofloxacin also carries this risk but may be less significant than the other two). Doxycycline is known to cause GI upset (nausea and vomiting), and it allegedly causes tooth graying/discoloration in juvenile animals. However, multiple sources claim that this is much less likely with doxycycline compared to its other tetracycline relatives, and many clinicians do not refrain from use due to this potential side effect. I personally have never seen this occur in any of my juvenile patients. The ACVIM Working Group on respiratory diseases states that doxycycline is safe and will not cause enamel discoloration in dogs and cats > 4 wks of age. Cats are particularly susceptible to oral ulceration and esophageal ulceration and stricture from doxycycline not being absorbed, so coating doxycycline with a safe lubricant before pilling and chasing it with a syringe of water (2-6 ml) immediately after is recommended. I have also seen evidence of a dog who sustained severe oral ulcerative lesions after he “hid” his doxycycline in his cheek pouch and never swallowed it. Chloramphenicol is a very effective bacteriostatic antibiotic that works well for Bordetella and Mycoplasma infections. It is banned in food animals because it is not approved for human use given its reports of blood dyscrasias and a disease known as “gray baby syndrome”. While rare, bone marrow suppression can occur even with a person making direct skin contact with a tablet, so I always have a cautionary conversation with owners before prescribing and send home gloves for administration. In homes where an owner may be pregnant, immunosuppressed, or have a pre-existing bone marrow condition, I tend to avoid this drug. Hepatic enzyme elevation can occur in dogs after taking chloramphenicol, and a dose-related myelosuppression has also been documented but is not as severe as what is documented in people and usually is only seen with prolonged use. Cats are particularly sensitive to chloramphenicol’s effects and are at higher risk of developing renal failure than other species. Chloramphenicol is a q 8 hr drug which can be difficult for owner compliance when factored in with needing to wear gloves for every administration. GI upset is also a commonly reported side effect.
Enrofloxacin (10 mg/kg q 24 hr) is a fluoroquinolone antibiotic that is attractive for several reasons. It is typically effective for most first-line infectious pneumonias (Bordetella/Mycoplasma) as well as for E. coli and other gram negative enteric organisms typically cultured in aspiration pneumonia. However, it does not provide a wide spectrum against certain gram + organisms (such as Streptococcus) and does not protect against anaerobes. Anecdotally, I also find enrofloxacin to be the least “upsetting” of the antibiotic choices in regards to GI side effects. Its once daily dosing is also a better option for owner compliance as it is a concentration-dependent drug. However, in large breed puppies, there is a risk of growth plate deformities under 8 months, and bacterial resistance to fluoroquinolones is becoming a more widespread and serious issue in both human and veterinary medicine. Due to the emergence of multi-drug-resistant organisms, I try to reserve enrofloxacin for select cases that are either not improving to initial therapy or have relative contraindications to other antibiotics. I am also very cautious with prescribing enrofloxacin in cats due to its potential for retinal damage. I try to use marbofloxacin (which in theory carries similar risks as enrofloxacin), but it may be slightly safer for cats.
Aminoglycoside therapy should be reserved for cases in which infectious pneumonia and sometimes aspiration pneumonia are not responsive to first-line treatments or if indicated by culture results. This class includes amikacin and gentamicin, with amikacin being the least nephrotoxic of the aminoglycosides. This class of antibiotics works particularly well on most gram negative bacteria, some gram positive bacteria, but does not treat anaerobes. Amikacin is more commonly used over gentamicin given the development of gentamicin-resistant bacteria. Typically, aminoglycosides would be administered at a referral hospital where concurrent IV fluids can be given and daily renal values and urinalyses can be performed to look for casts. Aminoglycosides are usually combined with another class of antibiotics (commonly a beta lactam which has synergistic antibacterial effects) to provide broader spectrum coverage for anaerobes and some gram positive bacteria, unless culture results indicate that aminoglycosides alone are sufficient.
Other treatments that may help clear airway secretions and stimulate immune system clearance include nebulization and coupage. In cases of severe alveolar infiltrates or complete lung consolidation, neb/coupage can serve to break up areas of inflammation and pus that antibiotics may never be able to fully penetrate. Nebulization can be performed in-hospital with a nebulizer or humidifier for 5-10 minutes every 6-8 hours. At home, owners can either place their pet in a steamy bathroom for 5-10 minutes 2-3 times per day for the first 1-2 weeks or have them sit in close proximity to a cold mist humidifier for 5-10 minutes. I personally do not coupage patients who have just had a laryngeal tieback procedure or in documented cases of excessive regurgitation or megaesophagus.
Bronchodilators are discussed in many sources about their potential use in pneumonia. There is no evidence to support their use in pneumonia, and some experts argue that opening airways further may allow infection to spread to naïve areas of lung. I very rarely reach for a bronchodilator and have only done so in cases of extreme distress while trying to buy a few minutes while setting up the ventilator or in cases where a pre-existing lower airway inflammation is known or suspected.
Steroids may have historically been controversial as part of the standard treatment for pneumonia, but in the recent years, there is no evidence to support its use and may actually contribute to comorbidities and worsening infection. The only scenario in which I would consider steroids is in a brachycephalic dog (or cat) who has aspirated and developed a severe partial upper airway obstruction from laryngeal edema due to coughing and regurgitation. In this particular scenario, I would only give a single dose for its anti-inflammatory effects on the upper airway.
Monitoring success of therapy
The time at which pneumonia is completely resolved is also a bit of a subjective area. Historically, we were trained to continue antibiotic therapy for up to 2 weeks after the most recent set of radiographs showed radiographic resolution of pneumonia (this equates generally to at least 4-6 wks of treatment). Recent discussion among criticalists and internists have challenged this theory as there is little to no evidence to support this recommendation. This means that we may be prescribing unnecessary antibiotics for an arbitrary length of time, which poses a greater risk for creating drug resistance and altered GI flora. I typically do not treat any case of pneumonia for less than 2 weeks, although people are prescribed much shorter courses of antibiotics for pneumonia currently in human medicine. I look forward to veterinary clinical trials in the next decade to hopefully provide more evidence to help guide our duration of therapy. I do repeat radiographs every 2-3 weeks, but often times I have patients who may have developed chronic scarring that may mimic residual pneumonia, and the actual diagnosis of fibrosis vs unresolved pneumonia again becomes subjective unless repeated airway sampling is selected. Alternatively, I have had cases radiographically that only show mild improvement in pulmonary infiltrates, and I have prescribed several weeks to months of antibiotic therapy. In patients who are still coughing or are not reportedly as energetic as they were prior to acquiring pneumonia, I may recommend a repeated ETW or BAL to make sure we are still treating the right pathogens. In extreme cases, an entire lobe may remain consolidated and unresponsive to therapy after months. This poses a risk for abscessation and necrosis, and in very rare circumstances, a lung lobectomy may be recommended if repeated radiographs are not showing any changes over a period of weeks to months.
Prognosis
In general, the prognosis for pneumonia can be good. Survival rates have been reported between 77-96%. Therefore, it is absolutely reasonable to treat pneumonia no matter how many lung lobes are affected on radiographs and how severely. Prognosis becomes more guarded when hypoxemia reaches the critical level at which mechanical ventilation is indicated or a clinician is concerned about patient fatigue from working too hard to breathe. In those cases, the prognosis is obviously more guarded simply due to the stress associated with transport to a facility where MV is offered. (At our hospital, we have a portable oxygen tank that we reserve for critical cases to transport to our sister hospital for MV). However, most of the time patients have a poor prognosis by the time they are put on a ventilator is because the decision was made too late in the course of their disease to ventilate. While patients with pneumonia who require MV carry a more guarded prognosis, this does NOT mean that treatment is hopeless, and patients CAN survive to discharge and make a full recovery after being on a ventilator. The sooner a clinician prepares for this possible next step in treatment the better the chances are for that patient. If you are unable to perform an arterial blood gas or get a reliable pulse oximetry reading on a patient but the “thought” of MV has entered your mind based on their clinical condition, there is a good chance that your patient may need it sooner than later. In these cases I strongly recommend calling a criticalist ASAP to discuss the case and to make recommendations.
Feline Pneumonia
Feline pneumonia is rarely described in the literature, and thus most of my information is extracted from a single study (Macdonald, et al. J Am Vet Med Assoc 2003). These authors reported an overall incidence of 0.1% of infectious pneumonia in a feline population over a 10-year period. Infectious pneumonia is more common than aspiration pneumonia in cats, and viral and fungal causes appear to be more common in cats than in dogs, although bacterial pneumonia still outnumbered other causes of infectious pneumonia in this study. FIP was also found in 9 of 11 cats. Herpesvirus and calicivirus are also suspected to be related to infectious pneumonia but cannot always be proven on diagnostics. Hematogenous pneumonia (infection acquired from bacteria that spread through the bloodstream) is also considered more common in cats than in dogs as a cause of infectious pneumonia and should be suspected in cases of known bite wounds or wounds of unknown origin. Parasitic causes of pneumonia are uncommon but documented and should be considered in cats that go outdoors. Leukograms and radiographs can vary in their patterns like they do in dogs, although interstitial patterns may be more common on radiographs in cats. If time allows we will go into a discussion about the limited literature that exists on feline infectious pneumonia in more detail.
References
- Proulx, et al. In vitro bacterial isolate susceptibility to empirically selected antimicrobials in 111 dogs with bacterial pneumonia. J Vet Emerg Crit Care 2014; 24(2): 194–200.
- Plumb, DC. Plumb’s Veterinary Drug Handbook: Eighth Edition. Stockholm, WI: Pharmavet Inc, 2015.
- Kogan, et al. Clinical, clinicopathologic, and radiographic findings in dogs with aspiration pneumonia: 88 cases (2004–2006). J Am Vet Med Assoc 2008;233:1742–1747.
- Kogan, et al. Etiology and clinical outcome in dogs with aspiration pneumonia:88 cases (2004–2006). J Am Vet Med Assoc 2008;233:1748–1755.
- Tart, et al. Potential risks, prognostic indicators, and diagnostic and treatment modalities affecting survival in dogs with presumptive aspiration pneumonia: 125 cases (2005-2008). J Vet Emerg Crit Care 2010; 20(3): 319–329.
- Epstein, et al. Airway microbial culture and susceptibility patterns in dogs and cats with respiratory disease of varying severity. J Vet Emerg Crit Care 2010; 20(6): 587–594.
- Radhakrishnan, et al. Community-acquired infectious pneumonia in puppies: 65 cases (1993–2002). J Am Vet Med Assoc 2007;230:1493–1497.
- Macdonald, et al. Clinicopathologic and radiographic features and etiologic agents in cats with histologically confirmed infectious pneumonia: 39 cases (1991–2000). J Am Vet Med Assoc 2003;223:1142–1150.
- Wingfield, et al. Arterial Blood Gases in Dogs with Bacterial Pneumonia. J Vet Emerg Crit Care 1997; 7(2):75-78.
- Viitanen, et al. Co-infections with Respiratory Viruses in Dogs with Bacterial Pneumonia. J Vet Intern Med 2015;29:544–551.
- Weese, et al. ACVIM Consensus Statement on Therapeutic Antimicrobial Use in Animals and Antimicrobial Resistance. J Vet Intern Med 2015;29:487–498.
- Lappin, MR, et al. Antimicrobial use Guidelines for Treatment of Respiratory Tract Disease in Dogs and Cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. J Vet Intern Med 2017;31:279-294.
About the author
|

 Dr. Katie Sakakeeny graduated from The Ohio State University College of Veterinary Medicine in 2005 and completed an internship at Tufts University the following year. Dr. Sakakeeny completed a 3 year Emergency and Critical Residency at Angell Animal Medical Center in 2009 and was board certified by the American College of Veterinary Emergency and Critical Care in November 2009. Following completion of her residency she was employed as a criticalist at Tufts Veterinary Emergency & Treatment Specialists in Walpole, MA until 2010. Dr. Sakakeeny joined the IVG network of hospitals in January 2011.
Dr. Katie Sakakeeny graduated from The Ohio State University College of Veterinary Medicine in 2005 and completed an internship at Tufts University the following year. Dr. Sakakeeny completed a 3 year Emergency and Critical Residency at Angell Animal Medical Center in 2009 and was board certified by the American College of Veterinary Emergency and Critical Care in November 2009. Following completion of her residency she was employed as a criticalist at Tufts Veterinary Emergency & Treatment Specialists in Walpole, MA until 2010. Dr. Sakakeeny joined the IVG network of hospitals in January 2011.